Oculis, a Swiss biopharmaceutical company specializing in ophthalmic treatments, is developing a peptidomimetic therapy aimed at protecting the optic nerve from inflammation. This therapeutic approach is currently undergoing evaluation in a Phase 2 clinical trial, reflecting Oculis’ commitment to addressing unmet needs in eye diseases.
Therapeutic Approach
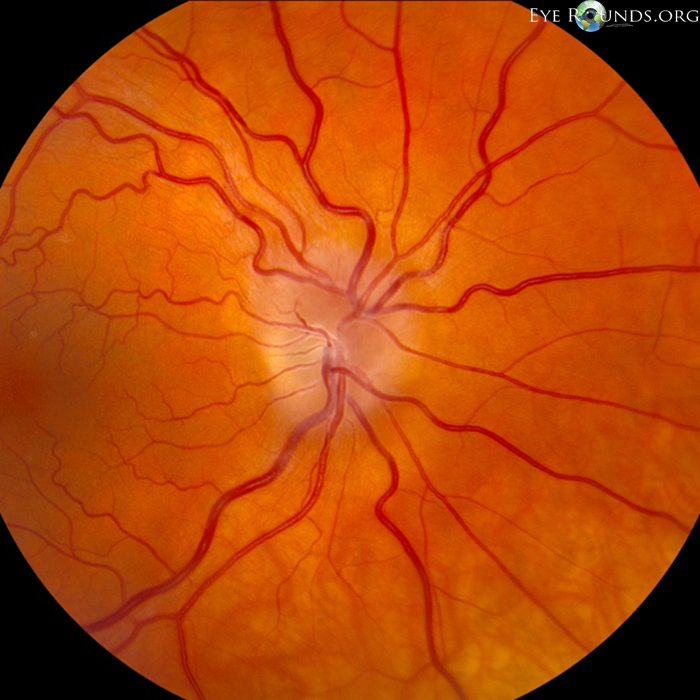
The investigational therapy utilizes a peptidomimetic—a small protein-like chain designed to mimic a peptide—that targets specific pathways involved in ocular inflammation. By inhibiting these inflammatory pathways, the treatment aims to preserve optic nerve function and prevent vision loss associated with conditions such as optic neuritis and other neuro-inflammatory disorders of the eye.
Clinical Development
Oculis has initiated a Phase 2 clinical trial to assess the safety and efficacy of this peptidomimetic therapy in patients experiencing optic nerve inflammation. The study is designed to evaluate the treatment’s ability to reduce inflammation, protect nerve fibers, and maintain visual acuity. Positive outcomes from this trial could pave the way for further clinical development and potential regulatory approval.
Innovative Drug Delivery
A key aspect of Oculis’ approach is the development of topical eye drop formulations that can deliver therapeutic agents effectively to the posterior segment of the eye. This non-invasive delivery method offers a patient-friendly alternative to intravitreal injections, potentially improving patient compliance and broadening the accessibility of treatments for retinal and optic nerve diseases.
Broader Pipeline
In addition to the peptidomimetic therapy for optic nerve inflammation, Oculis is advancing other ophthalmic candidates targeting conditions such as diabetic macular edema and dry eye disease. The company’s pipeline reflects a strategic focus on innovative treatments that address significant unmet medical needs in ophthalmology.
Conclusion
Oculis’ development of a peptidomimetic therapy for optic nerve inflammation represents a promising advancement in the field of ophthalmology. By targeting inflammatory pathways and utilizing patient-friendly delivery methods, the company aims to provide effective treatments for conditions that currently have limited therapeutic options. The ongoing Phase 2 trial will be instrumental in determining the future clinical and regulatory trajectory of this innovative therapy.