Moderna has received $590 million in funding from the U.S. Department of Health and Human Services (HHS) to expedite the development and trials of a bird flu vaccine. This investment underscores the government’s proactive efforts to bolster pandemic preparedness and combat emerging infectious diseases.
Background on the Bird Flu Threat
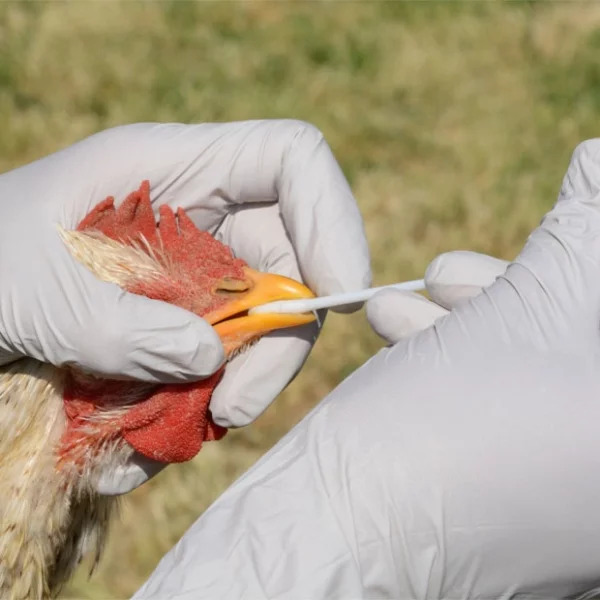
Avian influenza, commonly known as bird flu, poses a significant threat to both animal and human health. While primarily affecting poultry, certain strains of the virus have shown the potential to infect humans, raising concerns about a future pandemic.
- Recent Outbreaks:
- Highly pathogenic avian influenza (HPAI) outbreaks have devastated poultry populations worldwide, with sporadic human cases reported.
- Pandemic Potential:
- Experts warn that a highly transmissible strain could emerge, underscoring the urgency of developing effective vaccines.
Moderna’s mRNA Approach
Moderna will leverage its mRNA platform, which proved instrumental during the COVID-19 pandemic, to develop the bird flu vaccine. Key advantages of the mRNA technology include:
- Rapid Development:
- mRNA vaccines can be designed and manufactured more quickly than traditional vaccines, enabling faster responses to outbreaks.
- Versatility:
- The platform allows for targeting multiple strains of the virus, increasing the likelihood of broad protection.
- Scalability:
- Moderna’s existing manufacturing infrastructure can support large-scale production to meet potential demand.
Scope of the HHS Funding
The $590 million investment will be allocated to accelerate various aspects of the vaccine development process:
- Clinical Trials:
- Funding will support Phase 1 and 2 trials to evaluate the vaccine’s safety, efficacy, and optimal dosage.
- Manufacturing Expansion:
- Enhancements to Moderna’s production facilities will ensure readiness for large-scale vaccine manufacturing if required.
- Regulatory Support:
- Resources will be directed toward expediting the regulatory review process, ensuring the vaccine can be deployed swiftly if needed.
Implications for Pandemic Preparedness
The funding represents a significant step in strengthening the U.S.’s preparedness for future pandemics. Key benefits include:
- Enhanced Vaccine Arsenal:
- Developing a stockpile of bird flu vaccines will improve the ability to respond to outbreaks quickly.
- Global Leadership:
- The U.S.’s investment in cutting-edge vaccine technology positions it as a leader in global health security.
- Public Health Impact:
- Proactive measures could mitigate the human and economic toll of a potential bird flu pandemic.
Challenges Ahead
Despite the promise of mRNA technology, challenges remain:
- Virus Mutation:
- Rapid mutation of avian influenza viruses could complicate vaccine efficacy.
- Regulatory Hurdles:
- Ensuring swift but thorough regulatory approval will be critical to timely deployment.
- Global Coordination:
- Effective response will require international collaboration to address outbreaks beyond U.S. borders.
Conclusion
The HHS’s $590 million investment in Moderna’s bird flu vaccine development underscores the importance of proactive measures in pandemic preparedness. By leveraging its proven mRNA platform, Moderna aims to deliver a robust vaccine that could play a crucial role in averting a future health crisis.
For more information, visit Fierce Biotech.